Describe the Steps in Writing a Balanced Chemical Equation
3 Describe The Steps In Writing A Balanced Chemical. The subscripts tell you how many.
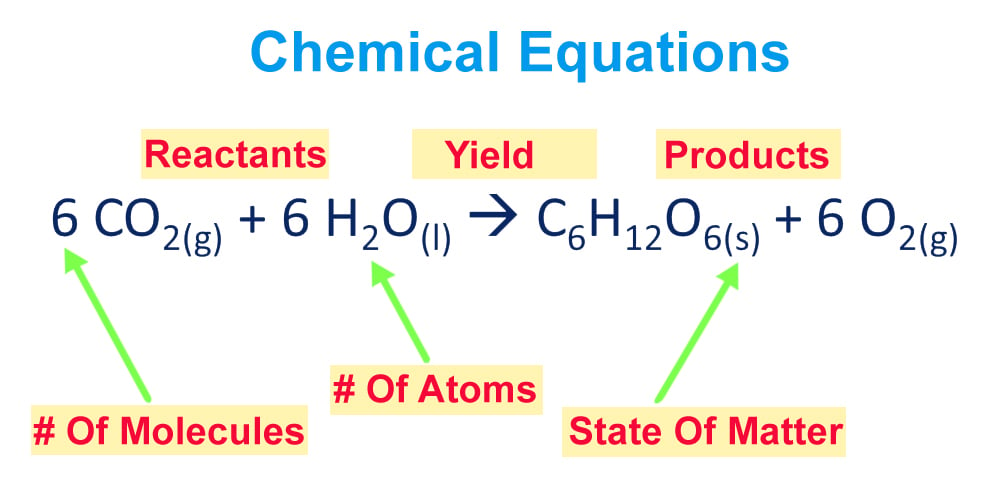
Balancing Chemical Equations How To Balance Chemical Equations
Guide for Reading Build Vocabulary Paraphrase Write the following chemi-cal equation on the board and have stu-dents provide synonymous words or phrases for each of the vocabulary.

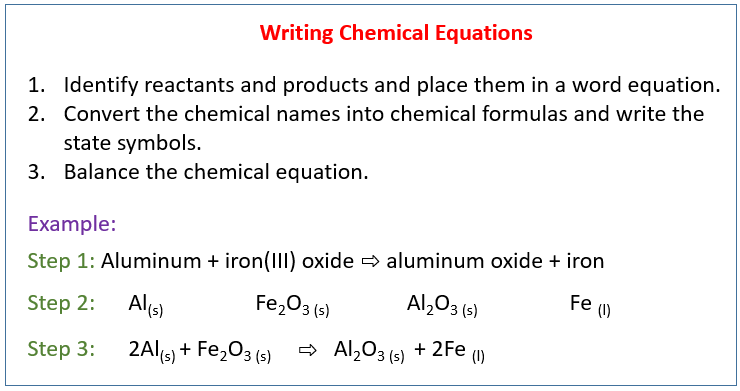
. The degrees of ionization decrease after. First step of ionization of sulfuric acid H 2 S O 4 a q H 2 O l H 3 O a q H S O 4 a q Second step of ionization for sulfuric acid H S O 4 a q H 2 O l H 3 O a q S O 4 2 a q b. Identify reactants and products and place them in a word equation.
H_3SO_4 aq 2NH_3 aq 2NH_4 aq SO. Consider as an example the decomposition of water to yield molecular hydrogen and oxygen. In a chemical equation there are subscripts and coefficients.
Solved Write A Balanced Chemical Equation For The Reaction Of Solid Strontium With Iodine Gas. 4 4 yes. Do a final check to make sure the equation is balanced.
The equation can now be written CH 4 O 2 CO 2 2H 2 O. Write down how many atoms of each element there are on each side of the reaction arrow. Identify the reactants and products.
A Heating copper II sulfide in the presence of diatonic oxygen produces pure copper and sulfur dioxide gas. 1 determine the correct formulas for all the reactants and products. HNO_3 aq NH_3 aq rightarrow NH_4 aq NO_3- aq Sulfuric acid reacts with ammonia in aqueous solution.
Include states of matter in all equations. If two or more reactants or products are involved separate their formulas with plus signs. Role of Graphite rod in electrometallurgy of Aluminium.
Describe the three steps involved in the leaching of bauxite to get pure alumina equations not expected. Then use a coefficients to balance the equations so that the same number of each atom appears on both sides of the equations. So what this means is that you just need to write all of the reactant and the products but you dont need to include their coefficients which means that once again its not yet balance.
An old way of writing it was FeOFe2O3 The balanced equation for production from the elements is - 3Fe 2O2 -. Sodium hydroxide ironII chloride sodium chloride ironII hydroxide. Click card to see definition.
Your diagram should include the reactants activation energy transition state and products. Place reactants on the left of the reaction arrow and products on the right. To write a balanced chemical equation first write the skeleton equation.
Include states of matter in the equations. Fe3O4 Commercially it is made by for example redung Fe2O3 with CO 3Fe2O3 CO. Write a balanced chemical equation including phases to describe the following reactions of sulfuric acid and nitric acid.
Add coefficients the numbers in front of the formulas so the number of atoms of each. Nitric acid HNO 3 reacts with ammonia NH 3 in aqueous solution. B When heated baking soda.
So the element by element comparison includes counting elements and then appropriately adding coefficients. Click again to see term. Write a balanced chemical equation including phases to describe the following reactions.
First write the skeleton equation. Sulfuric acid dissolves in water. Write skeleton equations for these reactions.
Write The Balanced Chemical Equation For Following Reaction Sodium Metal Reacts With Water To Give Hydroxide And Hydrogen. CH 4 2O 2 CO 2 2H 2 O. 2 2 4.
Place these in the proper order. Write a balanced chemical equation including the state symbols. Tap card to see definition.
2 write skeleton equation by placing the formulas for the reactants on the left and the formulas for the products on the right with the yields sign in between. Write a balanced chemical equation to represent the reaction. Describe the steps in writing a balanced chemical equation.
The idea is not balanced and your balance that I just put a three in front of their. Nitric acid reacts with ammonia in aqueous solution. Sulfuric acid dissolves in water.
The equation is completely balanced by showing two oxygen molecules four atoms as reactants. And then once you finished the final step once youve put all the coefficient and you think you counted each element by one by one. 1111 Describe how to write a word equation.
Easy Steps for Balancing Chemical Equations. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. Place them based on the chemical equation and write the state symbols.
Follow four easy steps to balance a chemical equation. If you simply verify or check final equation to make sure that. Write chemical equations for the involved reactions.
To write a balanced chemical equation first write the skeleton equations. Then use coefficients to balance the equation so that it obeys the law of conservation of mass. Use the sulfur-containing product formed in step 1 as the starting acid in step 2 it is asking to write both step 1 and 2 this is all the info I am given.
How to Balance a Chemical Equation Final Step 1. Basic Notation Used in Equations. Place the steps necessary to balance a chemical equation correctly in order starting with the first step at the top of the list.
So this question asked us to explain the steps of balancing chemical equations so the first step is to write the unbalanced equation. Tap again to see term. Convert the chemical names into chemical formulas.
How To Balance A Chemical Equation 7 Steps With Pictures Instructables. In order to balance the chemical equation you need to make sure the number of atoms of each element on the reactant side is equal to the number of atoms of each element on the product side. Write the unbalanced equation to show the reactants and products.
Adjust the coefficients such that there. Then use coefficents to balance the equation so that it obeys the law of conservation of mass. What are the steps for writing and balancing a chemical equations.
1112 Describe how to write a skele-ton equation 1113 Describe the steps for writing a balanced chemical equation. Draw an energy diagram similar in style to the one in question 1 of the reaction in which you label the energies of each step in the reaction and the activation energy. Determining and Balancing the First Element.
1 2 2 1 4. In order make both sides equal you will need to multiply the number of atoms in each element until both sides are equal. It is not yet balanced since there are only two oxygen atoms shown as reactants and four as products.
Sulfuric acid H 2 SO 4 reacts with ammonia in aqueous solution. Pick an element that appears in. Write a skeleton equation for heating copper II sulfide in the presence of diatomic oxygen.
Hydrogen gas Oxygen gas Water. Write a word equation. Write a balanced chemical equation including phases to describe the following reaction.
Writing A Balanced Chemical Equation Video Lessons Examples And Solutions

How To Balance Chemical Equations 11 Steps With Pictures

How To Balance Chemical Equations 11 Steps With Pictures Chemical Equation Balancing Equations Balancing Equations Chemistry
Comments
Post a Comment